Elevate Your Innovations with sRbumin®
Recombinant Human Albumin
for Various Technical Applications
Such as..
The New Standard of Excellence in Recombinant Human Albumin
Elevate Your Innovations with sRbumin®
The New Standard of Excellence in Recombinant Human Albumin
- Animal / Human Component Free
- Proven Clinical Safety
- Optimized Production for Cost Efficiencies
- Comprehensive Technical & Regulatory Support
Recombinant Human Albumin
for Various Technical Applications
Such as..
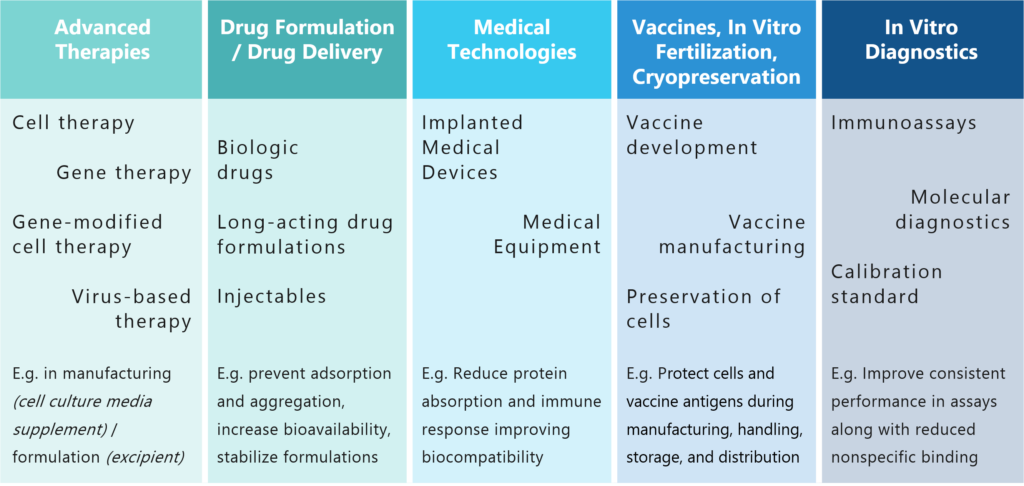
Advanced Therapies
(Cell & Gene Therapy and Tissue
Engineering Products)
Drug Formulation, Drug Delivery
(Biologics and Small Molecules)
Medical Technologies
(Implantable Medical Devices,
Medical Equipment, IVD)
Vaccines
(Manufacturing and
Formulation)
In Vitro Fertilization, Cryopreservation
(Protection of Cells)
Advanced Therapies
(Cell & Gene Therapy and Tissue
Engineering Products)
Drug Formulation, Drug Delivery
(Biologics and Small Molecules)
Medical Technologies
(Implantable Medical Devices,
Medical Equipment, IVD)
Vaccines
(Manufacturing and
Formulation)
In Vitro Fertilization, Cryopreservation
(Protection of Cells)
The New Standard of Excellence in Recombinant Human Albumin
In the ever-evolving landscape of life sciences, pharmaceuticals, biotechnology, and medical technologies the need for high-quality excipients and ancillary materials is paramount.
Why Choose sRbumin®
Facilitating a seamless transition from R&D to clinical and commercial-scale applications, backed by Shilpa Biologicals’ large production capacity in the range of metric tons.
Recombinant albumin with outstanding purity and high batch-to-batch consistency, 100% free from material of animal and human origin in its formulation and throughout the manufacturing process.
A clinical Phase 1 trial of sRbumin® has been successfully completed for direct medical application, with a Phase 3 trial planned for 2025; a drug master file (DMF) is available.
By optimizing every aspect of the production, we deliver cost efficiencies while maintaining the rigorous quality standards critical to success.
Our customer support solutions assist you at every stage of your process, helping you reduce complexities and improve efficiency in your path to market.

sRbumin®
Designed for an Array of Enhanced Technical Applications
Meticulously designed for various technical uses, sRbumin® is ideal for use in research & development and the formulation and manufacturing of, for example, (bio)pharmaceuticals, cell & gene therapies, vaccines, and beyond, such as the coating of implantable medical devices.
Ancillary material for advanced therapies, media supplement for cell culture – Recombinant human albumin can promote cell growth, viability and productivity across various cell types, supporting applications from R&D to large-scale bioproduction of advanced therapies.
Excipient for drug formulation – recombinant human albumin can serve as an excellent stabilizer and solubilizer for various drug formulations, enhancing their shelf life and efficacy.
Drug delivery vehicle – leverage the natural binding properties of recombinant human albumin to develop advanced drug delivery systems with improved pharmacokinetics and reduced toxicity.
The biocompatibility of recombinant human albumin makes it an excellent choice for coating implantable medical devices, as it may reduce the risk of adverse reactions and enhance device performance.
Recombinant human albumin can enhance the stability and efficacy of vaccines during production and storage
As a media component recombinant human albumin can improve culture conditions and increase success rates in assisted reproductive technologies as well as improve storage conditions and cell viability in cryopreservation of cells.
Meticulously designed for various technical uses, sRbumin® is ideal for use in research & development and the formulation and manufacturing of, for example, (bio)pharmaceuticals, cell & gene therapies, vaccines, and beyond, such as the coating of implantable medical devices.
For example, as ancillary material or media supplement for cell culture recombinant human albumin can promote cell growth, viability and productivity across various cell types, supporting applications from R&D to large-scale bioproduction of advanced therapies.
Excipient for drug formulation – recombinant human albumin can serve as an excellent stabilizer and solubilizer for various drug formulations, enhancing their shelf life and efficacy.
Drug delivery vehicle – leverage the natural binding properties of recombinant human albumin to develop advanced drug delivery systems with improved pharmacokinetics and reduced toxicity.
For example, recombinant human albumin’s biocompatibility can make it ideal for coating of implantable medical devices, potentially reducing the risk of adverse reactions and improving device performance.
For example, recombinant human albumin can enhance the stability and efficacy of vaccines during production and storage
As a media component recombinant human albumin can improve culture conditions and increase success rates in assisted reproductive technologies and improve storage conditions and cell viability in cryopreservation of cells.
sRbumin® Product Offerings
Product Range
sRbumin®
sRbumin® FAQ (Frequently Asked Questions)
Product & Purchasing
You may purchase sRbumin® directly from Shilpa Biologicals. Please contact our sales team at for pricing, availability, and sample requests.
sRbumin® is offered as a 20% (w/v) solution in volumes of 20 mL, 50 mL, and 100 mL, as well as bulk quantities. Lyophilized sRbumin® and custom packaging are available upon request.
Our sRbumin® liquid presentationhas a long shelf life of 36 months (from the date of manufacturing) when stored at 2–8°C (protect from light; do not freeze).
Manufacturing
sRbumin® is a recombinant human albumin (rHA) that is produced using a yeast-based expression system ensuring high batch-to-batch consistency and remarkable purity. sRbumin® is 100% free from animal and human components.
sRbumin® is free from the risk of blood-borne pathogens while delivering outstanding purity and unparalleled batch-to-batch consistency, compared with plasma-derived albumin. Our recombinant manufacturing process and large manufacturing capacity (in the range of metric tons) enables us to offer a dependable supply to our customers from R&D and clinical scale to commercial production
Regulatory, Quality & Clinical Safety
sRbumin® is manufactured in accordance with GMP requirements and undergoes stringent quality control for purity, sterility, and consistency.
Shilpa Biologicals can provide access to a Type II Drug Master File (DMF) filed with the U.S. FDA, along with quality documentation, including a certificate of analysis (CoA) and certificate of origin (CoO). A quality agreement can be readily implemented upon request.
sRbumin® has successfully demonstrated clinical safety in a Phase 1 clinical trial for direct therapeutic use. sRbumin® can be readily used as an excipient or ancillary material for various technical applications and therapeutic modalities from R&D to commercial products.
Use Cases
Yes, as ancillary material sRbumin® can support stem cell expansion, CAR-T cell production, and viral vector manufacturing, among other applications.
sRbumin® effectively binds small molecules, growth factors, cytokines, peptides, proteins, and other biologics, enhancing the stability of your assets. In addition, sRbumin® can improve bioavailability, extend product half-life, reduce unwanted toxicity and optimize the therapeutic window of your product
Yes, sRbumin® is designed for scalable use in bioreactors, upstream cell culture, and purification workflows.
Yes, sRbumin® is optimized for serum-free and chemically defined media formulations, making
Yes, sRbumin® is ideal for lyophilization and can also be provided in lyophilized form
Yes, sRbumin® binds and stabilizes proteins, reducing product loss through aggregation and adsorption in biologic formulations, vaccines, and drug delivery systems.
Yes, sRbumin® enhances cell attachment, viability, and growth, making it ideal for use in 3D scaffolds and regenerative medicine.
sRbumin® reduces osmotic stress and stabilizes membranes, improving post-thaw recovery in cell therapy applications.
Yes, we offer custom concentrations and buffer options to meet specific application needs. Please contact us for further details
Yes, we provide samples of sRbumin®. Please contact us to discuss your needs and request a sample.

Comprehensive Support for Your Success
Support Services around sRbumin®
At Shilpa Biologicals, we go beyond providing a high-quality product. Our team of experts offers a range of support services to help ensure your success.
Access to comprehensive quality and regulatory documentation, including GMP compliance certifications and drug master file (DMF) support.
Our team of experts is available to provide advice on:
- Application-specific optimizations
- Troubleshooting and problem-solving
- Scale-up strategies
Complimentary formulation development or optimization services* and access to our team of formulation scientists for consultation* at Shilpa Biologicals. (*Conditions apply)
Please get in touch for more details.
Complimentary custom conjugation development* and optimization of conjugation chemistry and conditions*. (*Conditions apply)
Please get in touch for more details.
Support for the development and manufacturing of recombinant human albumin fusion proteins.


The Shilpa Medicare Group
The Shilpa Medicare Group is a leading, innovation-driven pharmaceutical company and CDMO headquartered in India. It is recognized for its commitment to developing and manufacturing high-quality, affordable healthcare solutions.
Since its foundation in 1987, Shilpa Medicare has steadily expanded its expertise across developing and manufacturing oncology drugs, oncology and non-oncology active pharmaceutical ingredients (APIs) and intermediates, finished dosage formulations such as transdermal and oral dissolving films (ODFs).
Shilpa Biologicals
In response to the increasing demand for cutting-edge biologics and biosimilars, Shilpa Medicare launched Shilpa Biologicals in 2016 as a strategic initiative to enter these markets.
As a major business line, Shilpa Biologicals provides end-to-end CDMO solutions supporting NBEs and biosimilars development from gene and cell line development to GMP, drug product, drug substance, and commercial manufacturing. Product categories include antibody and non-antibody biologics as well as antibody-drug conjugates for human and animal health using mammalian and microbial platforms.
Beyond this, Shilpa Biologicals has used its expertise in R&D, manufacturing, formulation, and fill & finish to develop and offer recombinant human albumin for medical use and various technical applications. This includes, for example, applications as a critical excipient for drug formulation or ancillary material for advanced therapies.
Since their foundation both Shilpa Medicare and Shilpa Biologicals have established itself as trusted partners in the pharmaceutical, biotechnology and life science sectors worldwide.
Revenue CAGR (2020-2024)
Employees globally
Manufacturing sites
Latest Research
Our Latest Case Studies
Partnering
Shilpa Biologicals possesses the capacity and resources to collaborate with potential partners in advancing the development, characterisation, and commercialisation of novel formulations, drug conjugates, and albumin fusion proteins. We are eager to explore a variety of projects and opportunities for collaboration. Please get in touch for any enquiry.

Testimonial DEcide can We keep it
Our Happy Customers

Biology Student
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Lorem ipsum dolor sit amet, consec tetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua consectetur adipiscing.

Chemistry Student
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Lorem ipsum dolor sit amet, consec tetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua consectetur adipiscing.

Pharmacy Students
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Lorem ipsum dolor sit amet, consec tetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua consectetur adipiscing.

Physics student
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Lorem ipsum dolor sit amet, consec tetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua consectetur adipiscing.

Pharmacist Student
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Lorem ipsum dolor sit amet, consec tetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua consectetur adipiscing.





Get in Touch
Please feel free to reach out if you require further information or have any enquiries. We are here to assist you with any enquiries or questions you may have. If you are interested in obtaining a product sample, please also make use of the contact form.